
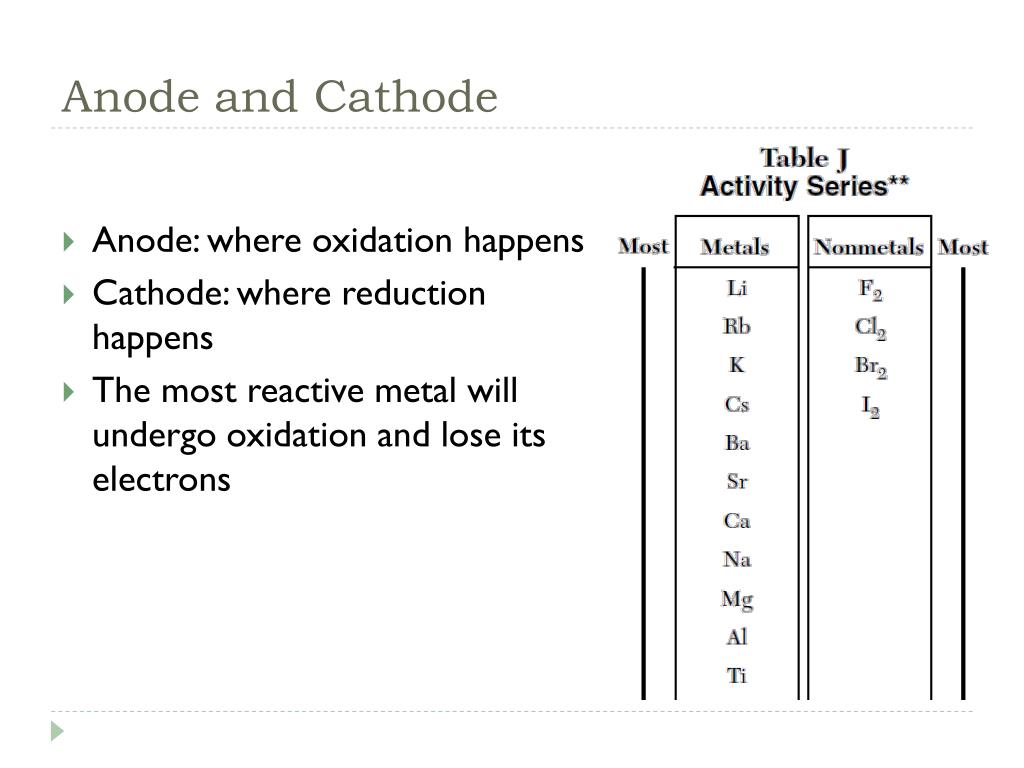
In a galvanic cell, electrons flow from the anode to the cathode, thus the anode is the metal with the lower standard potential, and the cathode is the metal with the higher standard potential. Electrons always flow from the metal with the lower standard potential to the metal with the greater standard potential. A more negative electrode potential means that the metal has a higher tendency to lose electrons. So how do we know which metal acts as the anode and which acts as the cathode? We can use the standard electrode potential of the metal, which is a measure of the metal’s tendency to lose electrons.
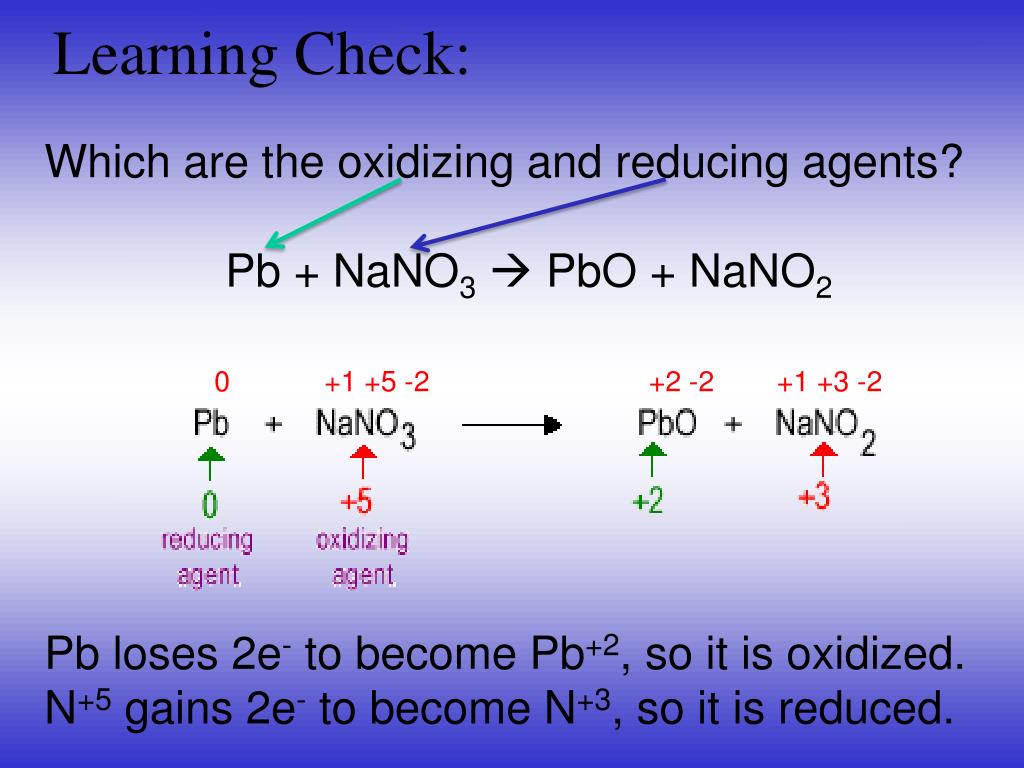
To help you distinguish between these two labels, remember the phrase 'red cat,' since reduction occurs at the cathode. The cathode is the electrode that gains electrons and is reduced. The anode is the electrode that gives up electrons in the reaction or is oxidized. The electrodes are connected by an external circuit, which the electrons travel through during the reaction. The solutions are connected by a salt bridge, which enables the flow of ions and maintains the charge neutrality of the solutions. The solutions consist of the ions needed for the half-reactions, either the oxidation or reduction reaction, that occur at each electrode. First, there are two metal electrodes, each of which is immersed into a different electrolyte solution. Some electrochemical reactions occur spontaneously, which we can study in a galvanic cell. To help you distinguish between the two, remember the phrase 'oil rig,' which stands for oxidation is losing, reduction is gaining. When it gains electrons, we say that it has undergone reduction or has been reduced. When a substance loses electrons, we say that it has undergone oxidation or that it has been oxidized. An electrochemical reaction is a process where electrons are transferred between two substances, with one losing electrons and the other gaining them.


 0 kommentar(er)
0 kommentar(er)
